If you’re leading healthcare MVP development, you’re not shipping a demo—you’re shipping the smallest unit of value and trust. That means day-one security, auditable claims, and realistic EHR paths, even if your first release is ruthlessly narrow.
The playbook here is lean with guardrails: prove one high-friction clinical workflow, instrument everything, and treat evidence like a first-class deliverable. Your edge isn’t feature breadth; it’s how fast you can learn without breaking privacy, safety, or credibility.
We’ll show you how to scope a production-grade MVP (not a disposable prototype), thread HIPAA and FDA constraints into actual velocity, and exit pilots with numbers buyers can defend. If “move fast and break HIPAA” makes you flinch (good), this guide is your conversion kit from opinion to outcomes—and from promises to purchase orders.
Key Takeaways
- MVP ≠ POC. Build a production-grade slice: RBAC, audit logs, basic interoperability, and clinical-context UX. You’re proving safety and outcomes, not just features.
- Process that scales under scrutiny. Map workflows first, run agile with compliance checkpoints, pilot with predeclared endpoints, and iterate on measured deltas—not vibes.
- Evidence beats pitch. Package results with data analytics, price to outcomes, and de-risk EHR paths; scale only when adoption is predictable and ops noise is down.
Table of Contents
- Understanding Healthcare MVP Development
- Pre-Development: Validating Your Healthcare Product Idea
- Essential Components of a Healthcare MVP
- Step-by-Step Healthcare MVP Development Process
- Regulatory Considerations for Healthcare MVPs
- Technology Choices for Healthcare MVP Development
- Cost Optimization Strategies for Healthcare MVPs
- Testing and Validation Strategies
- Launch and Go-to-Market Strategies
- Common Pitfalls and How to Avoid Them
- Scaling from MVP to Full Product
- How Topflight Can Help Build a Healthcare MVP?
Understanding Healthcare MVP Development
A healthcare MVP isn’t the cheapest demo—it’s the smallest unit of value and trust, shippable, supportable, and review-ready for real clinics. Here’s what makes that different.
What Makes Healthcare MVPs Different from Other Industries
In healthcare, an MVP isn’t a toy; it touches real patients, workflows, and risk. Beyond desirability/feasibility, you’re proving safety, auditability, and regulatory compliance from the first commit. That means:
- PHI handling
- access controls
- traceability
- basic interoperability
Unlike a prototype or proof of concept, a healthcare MVP must survive clinical reality: handoffs, downtime procedures, and data quality quirks. Success is measured by reduced risk and measurable outcomes, not just feature velocity.
In short: minimum viable product healthcare efforts are production systems with sharp edges removed.
The Lean Approach to Healthcare Innovation
Use lean startup tactics—but with guardrails. Write explicit hypotheses tied to clinical or operational outcomes (e.g., time-to-documentation, first-pass claim yield). Run small, consented pilots with real users and pre-approved data paths.
Keep scope razor-thin, but bake in logging, permissions, and rollback plans so learning doesn’t jeopardize trust. Treat product validation as evidence gathering: usability in clinic conditions, basic reliability under load, and compliance-ready documentation. Your backlog prioritizes risk burn-down alongside value delivery.
Common Misconceptions About Healthcare MVPs
- “MVP = cut corners.” No. Healthcare MVP development starts security on day one: encryption, audit logs, role-based access, and incident playbooks.
- “We’ll bolt on integrations later.” Plan the data model and FHIR shapes early so you don’t paint yourself into an EHR corner.
- “POC = MVP.” A proof of concept or throwaway prototype de-risks ideas; an MVP must be deployable, supportable, and review-friendly.
- “Compliance can wait.” Regulatory compliance is not a feature; it’s your license to operate and the cheapest risk you’ll ever buy if started early.
A strong healthcare MVP is the smallest unit of value and trust—engineered to learn fast without ever compromising patient safety or privacy.
Pre-Development: Validating Your Healthcare Product Idea
Before you build an MVP for healthcare, treat market validation as a clinical protocol: small, ethical, evidence-gathering loops that shape a digital health MVP people will actually use.
Identifying Real Healthcare Problems Worth Solving
Run disciplined user research with clinicians, nurses, admins, and patients. Map current workflows, handoffs, and failure points; quantify time, errors, and workarounds. Prioritize problems with high frequency/severity and clear patient engagement impact.
Output: a ranked problem list with hypothesized outcomes and acceptance criteria.
Market Research and Competitive Analysis
Inventory direct and adjacent competitors, including “do nothing / Excel” as a baseline. Note pricing, go-to-market, integrations, and proof claims. Validate reimbursement paths (codes, bundles, risk models) to de-risk product-market fit. Look for underserved niches or workflow gaps where incumbents underperform rather than trying to out-feature them.
Defining Your Target Users and Use Cases
Pick one primary user and one critical flow. For healthcare providers: define role, environment (clinic, virtual, inpatient), and constraints (EHR, device, bandwidth). For patients: motivation, literacy, and support network. Write one-sitting use cases: trigger → action → outcome → evidence captured. This keeps scope thin while maximizing patient engagement.
Regulatory Landscape Assessment
Decide early if you’re in wellness, clinical decision support, or SaMD territory. Sketch the minimal safeguards (HIPAA, audit logs, RBAC), data flows, and documentation you’ll need so healthcare app development decisions (cloud, frameworks, integrations) align with compliance and future certification paths. Plan for exportable evidence from day one.
Building a Business Case for Healthcare Organizations
Translate your hypothesis into dollars and risk: time saved per user, reduced denials, fewer readmissions, improved throughput. Frame a pilot with a narrow cohort, a 60–90-day runway, and unambiguous success metrics. Tie outcomes to existing budget lines to accelerate stakeholder buy-in. Your goal isn’t a pretty demo—it’s a believable path to operational value that a champion can defend.
Checkpoint: If a claim, a payer pathway, or an integration can’t be explained in one slide, it’s not validated. Keep the digital health MVP small enough to prove it fast.
Essential Components of a Healthcare MVP
A healthcare product MVP earns trust by doing a few critical jobs flawlessly, in the real mess of care delivery. Everything below keeps your healthcare software MVP small, safe, and shippable.
Core Features vs. Nice-to-Have Functionalities
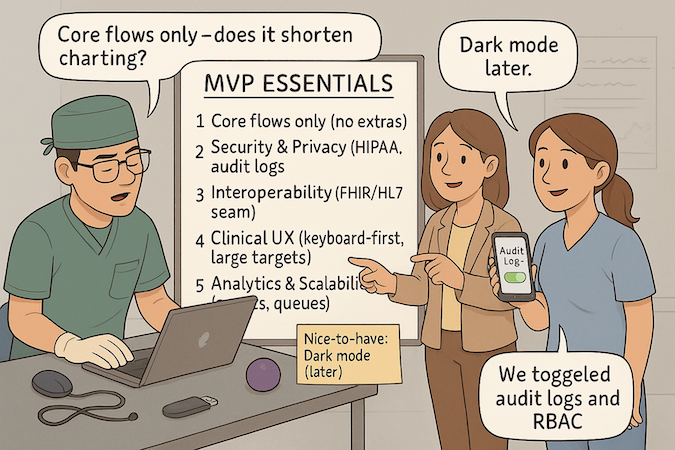
Ship the smallest workflow that moves a clinical needle—one role, one environment, one outcome. Core = the path through clinical workflows (capture → decide → act → document) plus uptime, audit, and support. Nice-to-haves (dashboards, themes, broad device support) wait.
Design for future scalability: stable domain model, idempotent APIs, and background jobs—no microservices tourism until the bottlenecks are real.
Security and Privacy Requirements from Day One
HIPAA compliant app development is not a phase. Default to least privilege, enforced data security (TLS 1.2+, encryption at rest with managed KMS), and explicit privacy boundaries. Required day-one controls:
- RBAC
- audit logs with immutable storage
- secrets management
- PHI data-flow diagrams
- incident runbooks
- BAAs with your cloud/services
Automate basic checks (SAST/DAST, dependency scanning) so security scales with feature velocity.
Basic Interoperability and Integration Capabilities
Plan interoperability even if you don’t integrate everything yet. Define patient/encounter identifiers, code systems (SNOMED/LOINC/ICD), and FHIR resource shapes for your core artifacts.
Build a thin integration seam: queue + retry + dead-letter, clear error codes, and a mapping layer that can evolve without refactors. Prove the seam with one authentic exchange (e.g., read-only Problems or Observations) and a mocked write to de-risk EHR directionality.
User Experience Design for Clinical Settings
Clinicians live in seconds, not minutes. Optimize for low handoffs, high signal, and offline tolerance. Patterns that fit clinical workflows:
- progressive disclosure
- keyboard-first forms
- clinical-safe defaults
- interruption recovery
For diverse patients, prioritize readable language, large tap targets, and assisted flows; accessibility is non-negotiable. If a screen doesn’t shorten a task or reduce errors, it’s noise.
Data Collection for Future Iterations
Instrument from day one. Capture event-level analytics (task completion time, error rates, drop-offs), operational metrics (queue depth, latency), and outcome proxies tied to your hypothesis.
Keep PII/PHI minimization in place and store raw events in a privacy-aware warehouse for reproducible analyses. Use the data to drive roadmap bets and capacity planning—the fastest path from MVP to product is measured learning, not guesswork.
Step-by-Step Healthcare MVP Development Process
Think of this as a controlled flight plan: the MVP development process sequences risk burn-down before scope creep, so you reach evidence fast without tripping compliance.
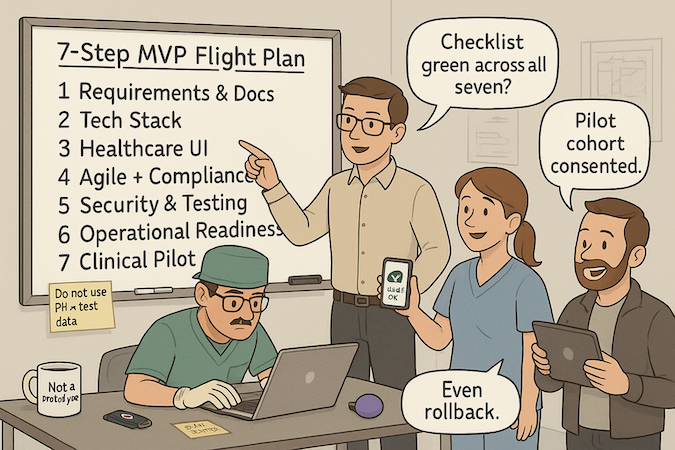
Here’s a quick map to build a healthcare MVP without drama: the steps below distill each phase’s purpose, outputs, owners, and “ready to move” checks—so you can scan, align, and execute.
| Step | Purpose | Key Outputs | Primary Owner(s) | Exit Criteria |
| 1) Requirements & Documentation | Align on clinical workflow and compliance scope. | Personas, workflow maps, user stories + acceptance, PHI/data-flow diagram, RBAC matrix. | Product Lead + Clinical SME | Approved spec and initial risk register. |
| 2) Technology Stack | Select a HIPAA-ready baseline you can support. | Reference architecture, managed services list, BAAs, environment plan. | Tech Lead + DevOps | Architecture review sign-off. |
| 3) Healthcare UI Design | Reduce cognitive load; design for interruptions. | Wireframes, interactive prototype, accessibility checklist. | UX + Clinician Reviewer | Usability sign-off on critical paths. |
| 4) Agile + Compliance Checkpoints | Deliver value with traceability. | Sprint backlog, test plan, story→code→test trace matrix. | Engineering + QA + Compliance | Sprint demo with documented evidence. |
| 5) Security Implementation & Testing | Harden the baseline continuously. | Threat model, SAST/DAST reports, pen-test scope & fixes, secrets/audit/immutability. | Security + Engineering | No high/critical vulns; controls verified. |
| 6) Operational Readiness & Release | Make shipping safe and reversible. | Release plan, runbooks, monitoring dashboards, rollback drills, go-live checklist. | DevOps + Support | Checklist passed; one-click rollback proven. |
| 7) Clinical Validation & Pilot | Prove outcomes in situ. | Pilot plan, data workbook, training, success metrics. | Product + Clinical Champion | Predefined KPIs met; decision doc for scale. |
Step 1: Requirements Gathering and Documentation
Start with workflows, not wishlists. Map actors, data flows, and failure modes; capture risks and mitigations. Write crisp user stories with acceptance criteria tied to clinical outcomes and auditing needs.
Document PHI touchpoints, RBAC, and log requirements. This creates a living spec healthcare teams can trust and review.
Step 2: Choosing the Right Technology Stack
Select HIPAA-ready cloud, managed services with BAAs, and frameworks your team can actually support. Favor boring, proven components over novelty. Healthcare app developers should define a reference architecture (auth, API, storage, monitoring) that preserves options for scale and integrations later—without overengineering now.
Step 3: Designing User Interfaces for Healthcare
Design for interruptions, time pressure, and cognitive load. Follow accessibility and (when applicable) medical-device UI patterns:
- clear hierarchy
- safe defaults
- error recovery
Usability in clinic conditions beats pixel art every time; prototype flows that reduce clicks, handoffs, and rework.
Step 4: Agile Development with Compliance Checkpoints
Run true agile development with a compliance spine. Each sprint: implement, verify, and evidence. Pair feature prioritization with risk reduction (security, safety, data quality). Keep traceability from story → code → test → artifact. Bake in lightweight documentation so audits don’t become archaeology.
Step 5: Security Implementation and Testing
Security is a feature you ship every sprint. Enforce least privilege, encrypt in transit/at rest, and threat-model new endpoints. Automate SAST/DAST and dependency scans; add:
- secrets management
- audit logs
- immutable storage
Gate merges via continuous integration checks and run periodic pen tests on critical paths.
Step 6: Operational Readiness & Release Management
Before any pilot, make production a button, not an adventure. Define the path (dev → staging → prod), release cadence, and rollback mechanics. Stand up on-call, support SLAs, and a go-live checklist that ops can run without engineering hand-holding.
- Environments and flags: feature-flag risky changes; practice blue/green or canary releases with a one-click rollback.
- Data safety: migration scripts with dry-runs, backups with periodic restore drills, and clear PHI retention/deletion policies.
- Observability: dashboards for latency/error budgets, alert routing, and runbooks tied to playbooks (who does what, when).
- Access and admin: least-privilege roles, audit exports, and admin guides/training so super-users can own day two.
- Compliance packet: release notes, known limitations, and evidence artifacts (tests, security checks) attached to each version.
Outcome: you can ship on schedule, revert safely, and support clinicians without heroics—making the pilot about outcomes, not firefighting.
Step 7: Clinical Validation and Pilot Testing
Prove it where it lives. Dry-run with de-identified data, then run a small, consented pilot. Track time-on-task, error rates, adoption, and outcome proxies.
Plan the development timeline as a sequence of learning sprints; schedule deliberate iteration windows after each pilot to fix what reality exposes. When you can demonstrate repeatable improvements, you’ve nailed MVP development for healthcare.
Regulatory Considerations for Healthcare MVPs
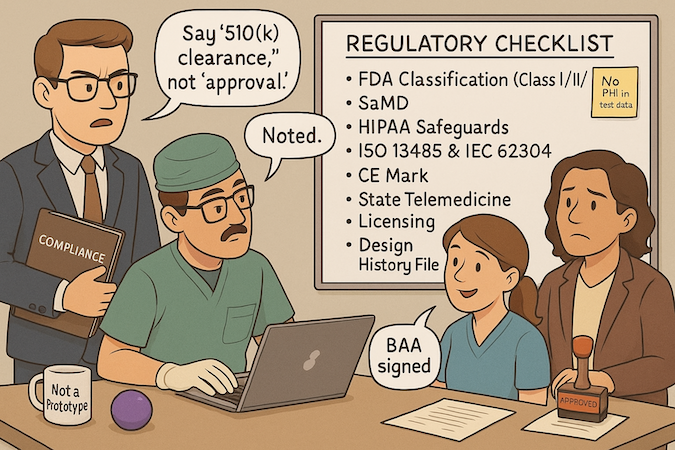
FDA Software Classification and Requirements
Start by classifying intent and risk, not code. If your MVP influences diagnosis or treatment decisions, you’re in software as medical device (SaMD) territory and must determine medical device classification (Class I/II/III).
Plan the lean path: Q-Sub (Pre-Sub) to de-risk questions, then 510(k) or De Novo as appropriate; reserve “FDA approval” language for PMA-class products. Treat a medical device MVP as evidence scaffolding:
- claims matrix
- hazards
- verification/validation plan
- update control for algorithms
HIPAA Compliance Essentials
Anchor privacy and security in workflow, not just tech. Map minimum-necessary data use, define lawful bases, and sign BAAs early. Implement administrative (policies, training), physical (facility/device safeguards), and technical (access control, auditability, transmission protection) measures so HIPAA is demonstrably baked into operations and procurement artifacts.
International Standards (ISO 13485, CE Mark)
If global is on your roadmap, lay QMS rails now. Stand up a lightweight ISO 13485 spine with design controls, risk management (ISO 14971), and software lifecycle governance (IEC 62304) to streamline CE Mark under MDR.
This prevents rework in medical device software development when you localize claims, labeling, and post-market surveillance.
State-Specific Healthcare Regulations
Telemedicine isn’t one rule; it’s 50. Confirm clinician licensure where patients are located, modality allowances (audio-only vs video), supervision rules, e-prescribing constraints, and data-location clauses in state contracts. Bake these into onboarding, scheduling, and consent flows so your rollout isn’t blocked by policy fine print.
Documentation Requirements for Future Scaling
Document as if someone skeptical will read it later—because they will. Maintain:
- design history file
- risk file
- requirements trace (risk → requirement → test → result)
- release notes
- clean audit trail of decisions
For a healthcare minimum viable product, this “evidence spine” lets you expand claims, pursue clinical trials or real-world evidence, and move markets without rewriting your process.
In short: keep the packet small, the claims precise, and the proof collectible from day one.
Technology Choices for Healthcare MVP Development
Pick a tech stack you can run in your sleep. The fastest way to create healthcare MVP value is a boring, supportable baseline you can audit and scale—then evolve only where reality demands it. Treat your MVP healthcare app like infrastructure, not a demo.
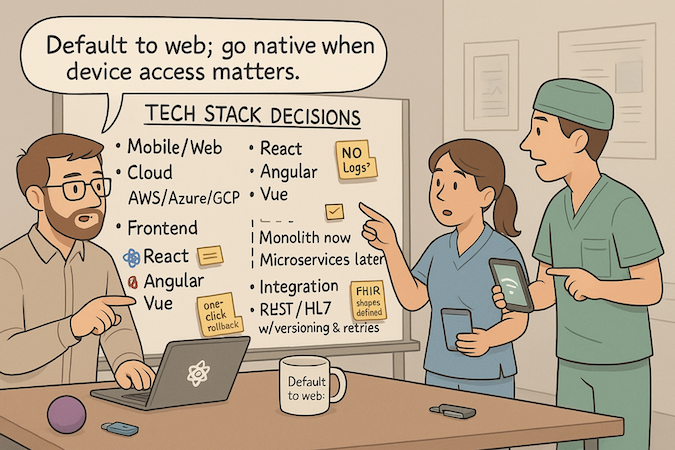
Mobile-First vs. Web-Based Approaches
If the primary tasks live at the point of care (camera, BLE, push, offline), go mobile; otherwise default to web for speed and distribution. Start responsive web to validate flows, then graduate to cross-platform (React Native/Flutter) for durable mobile needs. One critical flow per platform; no vanity parity.
Cloud Infrastructure for Healthcare
AWS, Azure, and Google Cloud all offer HIPAA-aligned building blocks; choose based on team skill and managed services you’ll actually use. Keep it simple:
- managed auth
- a single database
- object storage
- logs/metrics
Automate environments from day one so infra doesn’t bottleneck releases.
Frontend Frameworks for Medical Applications
Use what your team ships best: React, Angular, or Vue for frontend development. Favor design systems and form libraries over custom widgets; optimize for accessibility, keyboard-first input, and error recovery. Keep component boundaries clean so you can lift shared UI into mobile later without rewrites.
Backend Architecture for Scalability
Begin monolithic for speed and observability; carve out microservices only when a hotspot proves it (e.g., document processing, notifications). For backend development, standardize on
- REST (or GraphQL if you must)
- background jobs
- idempotent handlers
- structured logs
Contract tests protect integrations as you scale.
Integration Standards and APIs
Model data with FHIR/HL7 from the start, even if the MVP is read-only. Provide stable REST endpoints, versioning, retries, and dead-letter queues. Follow an EHR integration guide mindset: define resource shapes, identity mapping, and error codes before touching a sandbox. Prove one real exchange, mock the rest—reduce coupling until production demands it.
Cost Optimization Strategies for Healthcare MVPs
Treat medical MVP development like a clinical trial for spend: smallest budget that can produce defensible evidence.
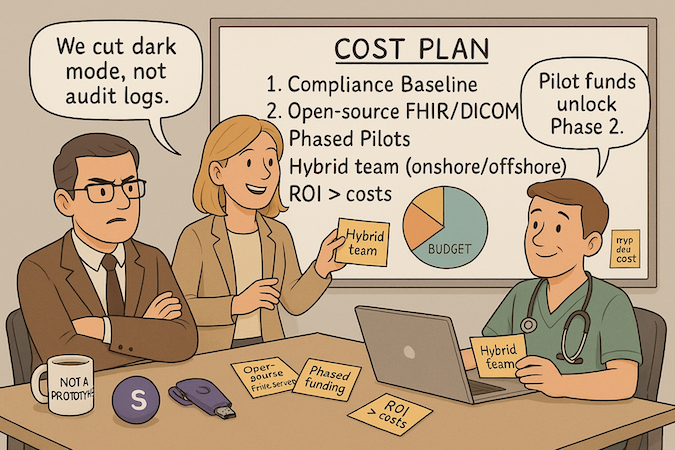
Budgeting for Compliance and Security
Ring-fence essentials first: access control, audit logs, encryption, BAAs, and pen-test scope. Allocate a fixed baseline before features—don’t raid it later. Track this as part of MVP development cost, not an “optional” line item; it’s the cheapest risk you’ll ever remove.
Using Open-Source Healthcare Tools
Leverage proven building blocks: FHIR servers (e.g., HAPI), DICOM libraries, and healthcare UI kits to accelerate without vendor lock-in. Validate licenses, threat-model dependencies, and contribute back where it reduces future maintenance. Open source is a cost-effectiveness lever and an innovation multiplier when paired with disciplined QA.
Phased Development Approach
Slice by outcomes, not by modules. Each phase funds a narrow hypothesis, a deployable feature, and a measurement plan. Gate the next tranche on results to control MVP development cost. This avoids big-bang overruns and keeps the backlog honest.
Offshore vs. Onshore Development Teams
Optimize for knowledge density. Use a hybrid model: onshore product/compliance leadership with offshore implementation pods for predictable execution. This lowers healthcare app development cost while preserving domain nuance and speed. Measure teams by working software and defect escape rate, not hourly rates.
Calculating ROI and Securing Funding
Define ROI in the language buyers use: first-pass yield, days in A/R, minutes saved per clinician, avoided readmissions. Tie each metric to one feature and one cohort; project payback windows and sensitivity ranges. Evidence like this:
- unlocks funding
- fuels pilots
- creates a competitive advantage beyond price
Frame the MVP as step one of digital transformation—a repeatable engine for measurable outcomes.
Bottom line: constrain scope, pre-pay your compliance debt, stand on open-source shoulders, and staff for learning speed. That’s how you control the development cost of an MVP without kneecapping outcomes or velocity—and how your MVP pays for itself in ROI instead of promises.
Testing and Validation Strategies
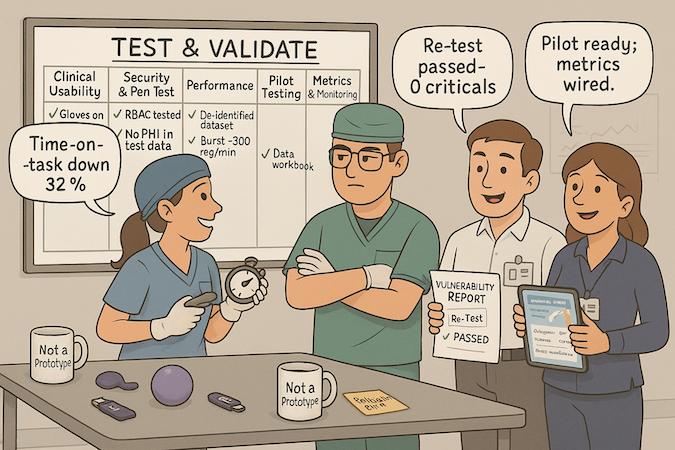
Clinical Usability Testing
Run task-based sessions under clinic-like constraints (interruptions, gloves, small screens). Follow FDA human-factors guidance to capture slips, recoveries, and time-on-task—then remediate before clinical validation escalates.
Security and Penetration Testing
Commission third-party assessments scoped to real data flows (auth, export, revocation). Schedule them before go-lives, not after; fix findings and retest so issues don’t surface during deployment.
Performance Testing in Healthcare Environments
Test on noisy networks, old hardware, and production-scale datasets. Simulate EHR/API latency, bursty queues, and failover. Passing on a MacBook Pro isn’t proof; passing in a busy clinic is.
Pilot Programs with Healthcare Partners
Treat pilot testing like mini trials: narrow cohort, pre-defined endpoints, and governance. Start with closed beta testing to de-risk onboarding and support. Graduating pilots should unlock evidence, not surprises.
Gathering Metrics for Product Improvement
Wire monitoring and telemetry to track adoption, errors, and outcome proxies; pair with structured user feedback (surveys, field notes). Decide in advance which metrics green-light the next iteration for your healthcare startup MVP/healthtech MVP—and which trigger redesigns. Good validation reduces rework and hidden app development costs more than any feature ever will.
Launch and Go-to-Market Strategies
Soft Launch with Selected Healthcare Partners
Treat soft launch as commercial dress rehearsal: procurement-ready docs, security questionnaires answered, and support SLAs in place. Limit access to 1–2 sites with clear success thresholds tied to patient outcomes and operational KPIs.
Building Evidence for Wider Adoption
Package outcomes with data analytics: baseline vs post-launch deltas, cohort definitions, and reproducible methods. Convert wins into a one-page evidence brief and a referenceable champion. This is your portable go-to-market strategy asset for scale.
- Snapshot the top three patient outcomes deltas (e.g., time-to-disposition, first-pass yield) and attach a reproducible data analytics workbook.
- Include a “how to implement” one-pager for healthcare IT (identity, data pathways, SLAs) to speed internal reviews.
Healthcare Sales Cycles and Decision Makers
Map the committee: clinical lead, finance, healthcare IT, compliance, and the buyer of record. Equip each with bespoke value props (safety for compliance, throughput for ops, dollars for finance). A credible healthcare MVP builder shows paths to integration and ongoing support, not just features.
Pricing Models for Healthcare Products
Price to outcomes and risk: pilot fee → annual subscription with volume tiers; optionally add performance-linked credits. Keep “all-in” totals transparent (implementation, training, support) to reduce friction for investors and buyers.
- Pilot-to-subscription credit if predefined patient outcomes targets are met.
- Tiered site/MAU/API pricing with clear implementation/training lines—clean unit economics for investors.
- Telemedicine module add-on with per-encounter or clinician-seat pricing aligned to telemedicine app development value drivers.
Marketing to Healthcare Organizations
Publish pragmatic content, speak at niche forums, and arm partners with ROI calculators. For telemedicine, emphasize continuity of care, staffing relief, and measurable patient outcomes. Make procurement easy: clear security posture, roadmap, and admin guides.
Common Pitfalls and How to Avoid Them
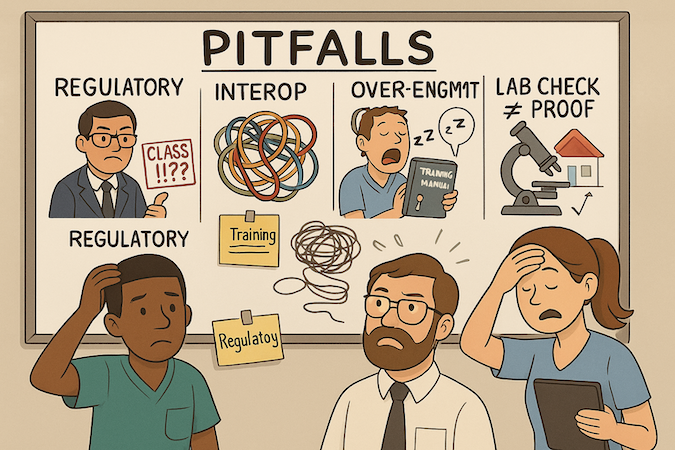
Underestimating Regulatory Requirements
If your MVP for medical product can sway diagnosis/treatment, treat claims as code: versioned, reviewable, and minimum-necessary. Assign an owner for each claim and risk. No “stealth” features. Small scope beats big promises—avoid the late-stage “surprise Class II” scramble.
Ignoring Interoperability Needs
Data shapes fossilize fast. Define identifiers, terminologies, and a mapping seam before the first integration ticket. Even if read-only, design for round-tripping later. Test error paths, not just happy flows. Interop debt compounds faster than feature debt—pay interest early.
Over-Engineering the MVP
Monolith first; carve out hotspots later. Resist premature AI integration or machine learning pipelines without curated data and acceptance gates. Pick one mobile path (web or React Native/Flutter) per use case; avoid chasing parity for vanity. Complexity that doesn’t de-risk outcomes is theater.
Neglecting Change Management
Clinicians don’t adopt slide decks. Budget for training, shortcut cards, super-user champions, and a rollback plan. Ship with admin controls, auditability, and safe defaults so ops can own day two. Change is a feature—treat it with the same rigor as code.
Insufficient Clinical Validation
Lab green checks aren’t proof. Run small, consented pilots with predefined endpoints and a stop/continue rule. Close the loop with field notes and fixes before scale. If results miss the mark, pivot deliberately: refine the cohort, narrow the claim, or kill the feature—don’t “hope” it in production.
Scaling from MVP to Full Product
When to Scale Beyond MVP
Scale when your healthcare product MVP delivers repeatable outcomes across sites and ops noise is trending down.
- Two+ sites hitting the same KPIs with similar staffing.
- Support tickets per active user dropping sprint-over-sprint.
- SLAs met for 4–6 weeks and on-call stable.
Adding Advanced Features
Prioritize compounding value over surface area. For remote monitoring, harden ingestion (queues/retries), tune alert thresholds, and expose clinician-facing summaries. Extend the web application first; introduce native apps only when device access, latency, or offline capture demand it.
- Ship offline-first sync for field use; resolve conflicts deterministically.
- Add role-aware automation (e.g., prefilled orders, smart defaults).
- Outcomes reporting with cohort filters, not vanity charts.
Expanding to New Markets
Translate claims, not just UI. Localize codes, reimbursement logic, and privacy notices; validate residency and consent early. A mobile first rollout helps field teams and patients, while admin controls remain on web for speed.
- Pilot one region/provider type with a single measurable promise.
- Pre-clear data-sharing agreements and identity mapping.
- Prepare support scripts and escalation paths before launch.
Building for Enterprise Healthcare
Enterprises buy risk reduction and operability. Ship SSO (SAML/OIDC), granular RBAC, audit exports, and real-time health dashboards; partition tenants and enforce rate limits.
- One-click environment spins; blue/green deploys with auto-rollback.
- Evidence packet: security posture, architecture diagrams, BAAs.
- Observability: traces, structured logs, uptime SLOs per tenant.
Balance time to market with operational maturity: scale when onboarding a large site requires configuration—not heroics.
How Topflight Can Help Build a Healthcare MVP?
You bring the clinical problem; we bring the rails. Topflight pairs senior product/engineering with compliance-first delivery, then accelerates with Specode—our AI healthcare MVP builder using reusable HIPAA-ready components to bootstrap core modules while you keep code ownership.
What We Deliver (Fast):
- Prototype in ~1 month. Investor-ready, deployed, and wired to real data flows—so you’re proving, not pitching.
- Deep EHR integration. SMART on FHIR, HL7v2, marketplace onboarding (Epic/Athena), and Mirth-powered mapping to fit real clinical workflows.
- AI that survives audits. LLM/RAG pipelines, ML models, and MLOps with measurable impact (documentation, decision support, revenue cycle, patient engagement).
- Production ops from day one. DevOps pipelines, observability, secure cloud infrastructure, and staged environments—no “rebuild for prod” tax.
- Clean connective tissue. Standards-first API development and evented integrations so partners can plug in without brittle contracts.
How We Work:
- Narrow problem → measurable claim → pilot with evidence.
- Security/compliance woven into delivery, not stapled on.
- Roadmaps that de-risk EHRs early and scale only where reality demands it.
If you’re ready to turn a sketch into a safe, shippable product—without losing speed—we’ll stand up the stack, wire the data, and get you to outcomes. From discovery through DevOps, API development, EHR integration, and secure cloud infrastructure, we own the path to evidence-backed healthcare MVP development.
FAQ
What's the fastest way to tell if our idea is MVP-ready?
Validate one painful workflow with stakeholder interviews, map the handoffs, and define a single measurable outcome; if you can’t explain it on one slide, it’s not ready.
Do we need HIPAA controls in the very first sprint?
Yes—least privilege, encryption, audit logs, and BAAs are non-negotiable guardrails, not backlog items.
Mobile-first or web for V1?
Default to web for speed; go mobile (or cross-platform) only if the core flow needs device access, offline capture, or push-driven moments.
When should we plan EHR integration?
Immediately at the data-model level—define FHIR shapes/identifiers now; prove one read path, mock the rest until production demands writes.
How do we keep costs in check without cutting corners?
Ring-fence compliance, use proven open-source blocks, and fund work in phased hypotheses with go/no-go metrics for each tranche.
What qualifies us to scale beyond MVP?
Repeatable outcomes across sites, falling support tickets per active user, stable SLAs, and a clear onboarding playbook.
What's a credible pilot exit package?
One-page outcomes brief, reproducible analytics workbook, a referenceable champion, and a documented path for healthcare IT to implement.