Wading through SaMD regulatory processes may sound dull, time-consuming, and expensive. And it should be. After all, it’s natural to expect more from healthcare apps that qualify as medical devices. The potential to save lives comes with greater responsibility.
So, if you’re a founder or a product manager striving to figure out a way to bring your healthcare app up and running in a regulatory-compliant way, I can feel your pain. It doesn’t have to be this way, though.
In this blog, we’ll discuss everything you need to know to make relevant modifications to your healthcare app to ensure SaMD compliance and successfully undergo the software as a medical device approval process.
As you understand, mastering the SaMD regulatory approval process gives you a competitive edge in this rapidly growing market, making your product more appealing to customers and investors.
Top Takeaways:
- Understanding SaMD: Software as a Medical Device (SaMD) represents an innovative intersection of healthcare and technology. It is software designed for medical purposes without being part of a physical medical device. This new frontier opens up vast opportunities for healthcare tech, but it also presents unique regulatory challenges.
- Navigating Regulatory Approval: The regulatory approval process for SaMD can be intricate, requiring compliance with regulations set by authorities such as the FDA. This SaMD regulatory approval process involves comprehensive documentation, rigorous testing, and detailed submission of necessary information. Despite its complexity, it’s a critical step in ensuring the safety and effectiveness of SaMD products.
- Mastering the Process: Mastery of the SaMD compliance and regulatory requirements demands a deep understanding of applicable regulations and a commitment to staying updated with changes or updates. Following best practices and seeking expert guidance can be instrumental in navigating around the software as a medical device approval process successfully.
Table of Contents:
- The Rise of Software as a Medical Device (SaMD)
- Understanding SaMD: What It Is and Why It’s Important
- Navigating U.S. SaMD Regulations: A Focus on FDA Guidelines
- Mastering the SaMD Certification Process: A Step-by-Step Guide
- Step 1: Laying the Regulatory Groundwork Before Development
- Step 2: Understanding Your Product Classification
- Step 3: Developing a Regulatory Strategy
- Step 4: Testing and Validation: Ensuring Safety, Effectiveness, and Usability
- Step 5: Documenting Your Journey: The Importance of Compliance Monitoring
- Step 6: Completing and Submitting the SaMD Registration Application
- Step 7: Post-Market Surveillance
- Challenges in SaMD Certification: Common Roadblocks and How to Overcome Them
- Learning from Success: a SaMD Case Study
- Looking Ahead: The Future of SaMD Certification
The Rise of Software as a Medical Device (SaMD)
The use of technology in healthcare has been on the rise for years, and it’s showing no signs of slowing down. With advancements in AI, machine learning, and big data analytics, healthcare apps are becoming more sophisticated in their ability to diagnose, monitor, and treat patients.
But with this increased reliance on technology comes a need for regulation. Enter SaMD – a new category of medical “devices” that are actually software used for medical purposes. These medical applications pose unique challenges when it comes to regulatory approval, as they blur the lines between traditional medical devices and software.
The Increasing Role of SaMD in Modern Healthcare
The use of SaMD is becoming increasingly prevalent in modern healthcare. From telemedicine apps that allow doctors to monitor patients remotely to AI-powered diagnostic tools for early disease detection, SaMD is revolutionizing the way we approach healthcare.
Reports indicate that the market for software as a medical device (SaMD) is poised to witness remarkable growth, with a compound annual growth rate of 51.88% projected from 2023 to 2028. This substantial growth can be attributed to the increasing demand for advanced technological solutions in the medical field.
SaMD offers a range of benefits over traditional medical devices, including cost-effectiveness, scalability, and real-time monitoring capabilities. With the potential to improve patient outcomes and reduce healthcare costs, it’s no wonder why the use of software as a medical device is on the rise.
Why Understanding SaMD Certification Matters
As a product manager or founder of a healthcare app, you may be wondering why understanding SaMD regulatory processes matters. Simply put, without proper software as a medical device certification, your app cannot be marketed as a medical device in the U.S. This means your app will not be able to reach its full potential and attract customers or investors.
Furthermore, SaMD certification ensures that your product meets strict safety and effectiveness standards, providing reassurance to both healthcare professionals and patients.
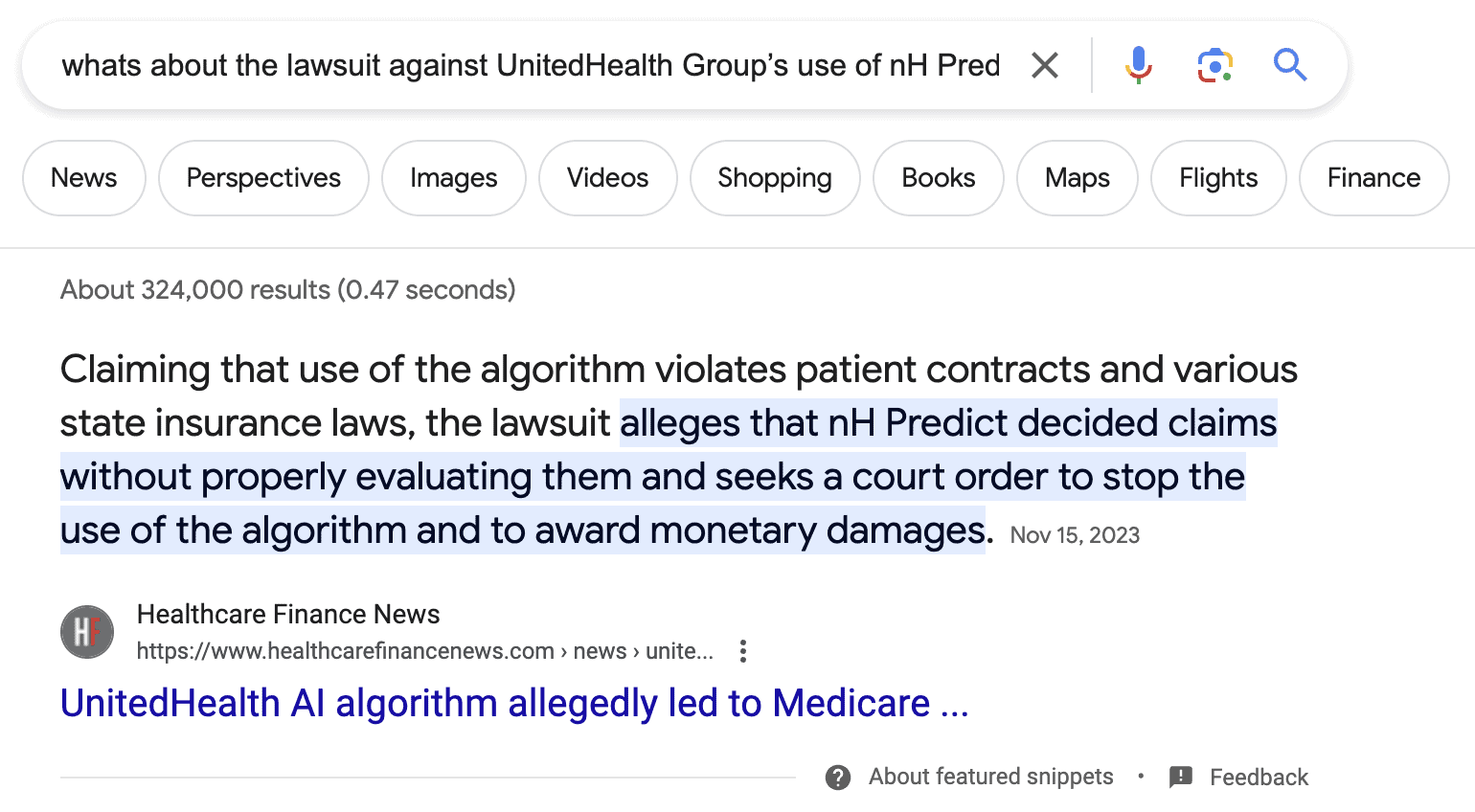
Of course, we definitely don’t want to find ourselves in this situation:
A quick search reveals that the AI app in question doesn’t have FDA clearance, which if it had, would require more efforts from the developer’s side. That would protect the company from potential lawsuits due to the app’s AI engine’s negligent work.
Understanding SaMD: What It Is and Why It’s Important
In the simplest terms, Software as a Medical Device (SaMD) refers to any software product intended to be used for medical purposes without being part of a hardware medical device, including:
- prevention
- diagnosis
- cure
- mitigation
- treatment of a disease
One may think that software as a medical device must be a stand-alone application, not connected to any hardware, working on its own. However, this is not true. SaMD may work in tandem with:
- other medical or general-purpose software
- smart sensors or medical hardware devices
- other SaMD
To qualify as SaMD, it is crucial that the software does not directly control or power the medical functions of integrated hardware or software. Instead, it utilizes data from such external sources to execute its medical features, in which case the SaMD compliance and regulatory requirements apply to such applications.
Read more on medical device integration
Does My Health App Qualify as SaMD?
In order to determine if your app falls under the SaMD category, it is important to establish the intended use and indications for the use of your product.
The intended use refers to the purpose of your device and what it will be used for.
Indications for use encompass the diseases or conditions that your device will diagnose, treat, prevent, cure, or mitigate. It specifies the target audience and the reasons for using the device.
Here are some helpful guidelines to help you identify SaMD:
- runs on general-purpose computing platforms (non-medical devices): smartphones, smartwatches, laptops, tablets, etc.
- is not installed directly on the hardware
- does not control hardware mechanisms
- connects to a hardware medical device but is not necessary for the device to fulfill its intended medical purpose
Examples of health apps that require software as a medical device certification:
- an AI-powered MSK therapy app that doesn’t use any external hardware except what’s available on a smartphone
- an app that syncs with a wearable weight management device
- a light-therapy app working in tandem with red and infrared LEDs
A clear example of not SaMD: a skin lesion detection scanner with AI algorithms running right on-device. Software powering the device renders the scanner useless if removed and is what they call Software in a Medical Device (SiMD) or embedded software (also, firmware).
NB SiMD also must undergo software as a medical device approval process but with some peculiarities.
If such a scanner would send all data to software installed on a PC or smartphone for analysis, the latter would qualify as SaMD and fall under the scrutiny of the SaMD regulatory approval process.
Also Read: A Complete Guide to EHR Integration
Categories of SaMD: A Breakdown
SaMD software can be broadly categorized into three classes according to the FDA guidelines: Class I, II, and III, depending upon the risk associated with their intended medical purposes.
Class I devices pose the least risk and typically include health and wellness apps like fitness trackers, while Class III devices pose the highest risk and include software used to support or sustain human life.
Class II lies in between and includes software like glucose monitoring apps. Understanding these categories is crucial as it influences the SaMD compliance and regulatory requirements your software must meet to achieve the software as a medical device certification.
The Impact of SaMD on Patient Care and Healthcare Efficiency
SaMD is transforming healthcare delivery by providing clinicians and patients with powerful tools to manage health outcomes. For instance, remote patient monitoring apps allow for continuous monitoring of patients’ vital signs, improving patient care, and reducing hospitalization times.
Similarly, predictive analytics software can analyze patient data to predict health risk-based conditions, enabling early interventions. Moreover, SaMD software also promotes healthcare efficiency by automating routine tasks, allowing healthcare providers to focus more on patient care. Overall, the advent of SaMD is ushering in a new era of personalized, efficient, and high-quality healthcare.
The bottom line? Making sense of all the SaMD regulatory processes is absolutely worth your time if you envision an innovative healthcare app.
Navigating U.S. SaMD Regulations: A Focus on FDA Guidelines
The U.S. Food and Drug Administration (FDA) plays a critical role in regulating the development and marketing of SaMD products. The FDA recognizes that software can be a medical device, but it’s not always easy to determine if an app should fall under this category.
To help companies navigate the complex SaMD certification process, the FDA has provided guidance on regulatory requirements for different types of software as a medical device. These guidelines provide a roadmap for companies to follow to ensure their app meets all necessary regulatory standards of Software as a Medical Device (SaMD) products.
The FDA also coordinates its efforts with international bodies such as the International Medical Device Regulators Forum (IMDRF) to promote global harmonization in SaMD regulations. This collaboration helps align regulatory practices worldwide, providing clearer paths for companies to market their SaMD products internationally.
Decoding FDA Classifications for SaMD: Class I, II, and III
Understanding how the FDA classifies SaMD is vital for navigating the SaMD regulatory approval process. The FDA categorizes medical devices into three classes of software based on the level of control necessary to assure safety and effectiveness:
- Class I: Devices in this category pose the least potential harm. Examples could include simple apps that provide general wellness advice.
- Class II: These devices have a higher risk profile and require more stringent controls. An example might be an app that calculates the dosage of a prescribed medication.
- Class III: The highest risk category, these devices often support or sustain human life, are of substantial importance in preventing impairment of human health, or present a potential, unreasonable risk of illness or injury.
The International Medical Device Regulators Forum (IMDRF) categorizes Software as a Medical Device (SaMD) into four categories based on the seriousness of the condition and the intended medical purpose of the software.
It’s essential to consider IMDRF classifications as well as local regulations like the FDA when planning your product rollout, especially if you’re considering entering foreign markets such as the EU (check out EU MDR specifically). Navigating the nuances of different SaMD regulatory processes can be complex, but understanding these classifications can provide a competitive advantage and facilitate a smoother approval process.
- Category IV represents SaMDs with the highest impact. These provide information to treat or diagnose critical conditions or diseases.
- Category III includes SaMDs that deal with serious situations, whether they’re used for treatment, diagnosis, or driving clinical management.
- Category II covers SaMDs of medium impact. They could be used in non-serious situations for treatment or diagnosis but also in serious or even critical situations to drive or inform clinical management.
- Category I SaMDs have the lowest impact, providing information to drive or inform clinical management in non-serious situations or serious ones.
| State of Healthcare situation or condition | Significance of info provided by SaMD to healthcare decision | ||
| Treat or diagnose | Drive clinical management | Inform clinical management | |
| Critical | IV | III | II |
| Serious | III | II | I |
| Non-serious | II | I | I |
Each class has different regulatory controls and premarket review pathways. It’s crucial to correctly classify your SaMD to understand the regulatory requirements you’ll need to meet in order to complete the software as a medical device approval process successfully.
The Role of the FDA in Regulating SaMD: A Deep Dive
The US FDA’s role in regulating SaMD is multifaceted. They establish and enforce regulations that ensure the safety and effectiveness of medical devices, including SaMD. This includes everything from premarket submissions to post-market surveillance.
For SaMD developers, understanding the FDA’s premarket submission process is essential. This involves submitting a Premarket Notification 510(k) if your device is equivalent to a device already on the market or a Premarket Approval (PMA) if your device is new or high risk.
The FDA also provides guidance for SaMD developers. Its guidance on premarket submissions document issued in June 2023 focuses on software functions in devices. Another document relevant to the SaMD compliance and regulatory requirements presents a proposed framework for risk categorization and corresponding considerations for SaMD.
To ensure quality management, the IMDRF has provided a Quality Management System specifically for SaMD. This system can be instrumental in ensuring that your SaMD meets international standards.
Lastly, the clinical evaluation of SaMD is a critical aspect of the FDA’s regulatory process. This involves assessing and analyzing clinical data (obtained during clinical trials) pertaining to a medical device to verify its clinical safety and performance.
With these resources and processes, the FDA aims to ensure that SaMDs are safe and effective for their intended uses, fostering innovation while protecting public health.
Mastering the SaMD Certification Process: A Step-by-Step Guide
Here’s a step-by-step list of all the stages as you go through the SaMD regulatory process:
- Step 1: Laying the Regulatory Groundwork Before Development
- Step 2: Understanding Your Product Classification
- Step 3: Developing a Regulatory Strategy
- Step 4: Testing and Validation: Ensuring Safety, Effectiveness, and Usability
- Step 5: Documenting Your Journey: The Importance of Compliance Monitoring
- Step 6: Completing and Submitting the SaMD Registration Application
- Step 7: Post-Market Surveillance
Navigating the software as a medical device certification process can seem daunting, but with a clear understanding of the steps involved, you can confidently lead your healthcare app through to regulatory acceptance.
Step 1: Laying the Regulatory Groundwork Before Development
Before you even start developing your application, it’s crucial to understand the intricacies of the software as a medical device approval process. Why? Because designing and architecting a healthcare app with regulatory requirements in mind from the get-go can save you significant time and resources down the line.
Retrofitting an existing app to meet the FDA’s SaMD compliance and regulatory requirements can be a lengthy and costly process (learn more about healthcare app development cost). So, how can you set yourself up for success?
Start by familiarizing yourself with the FDA’s guidelines on SaMD. Understand what class your product falls under and what that means in terms of applicable SaMD regulatory processes. Also, consider the Quality Management System (QMS) guidelines provided by the IMDRF, as this can be instrumental in ensuring your SaMD meets international standards.
Remember, a well-informed software development process is a streamlined one. By laying the regulatory groundwork before development, you’ll be better prepared to navigate the SaMD regulatory process and bring your healthcare app to market more efficiently. So, pick your provider of healthcare app development services wisely.
Step 2: Understanding Your Product Classification
As mentioned earlier, properly classifying your SaMD is crucial for understanding the regulatory requirements you’ll need to meet. This step involves analyzing the intended use of your application and its risk profile.
The SaMD regulatory approval process starts with identifying:
- Intended Use / Intended Purpose
The manufacturer’s objective intent for how a product should be used, as stated in their specifications, instructions, and information. This should also describe the significance of the information provided by the SaMD to healthcare decisions and the current healthcare situation or condition for applying SaMD.
- Medical Purpose
This includes activities such as diagnosing, preventing, monitoring, treating, or alleviating diseases and injuries; investigating, replacing, modifying, or supporting anatomy or physiological processes; supporting or sustaining life; controlling conception, disinfecting medical devices, and providing information through in vitro examination of human body specimens.
If you’re unsure about how to classify your product, consider consulting with a regulatory expert or utilizing self-assessment tools provided by the FDA and IMDRF. They will help you get through the maze of SaMD regulatory processes.
Step 3: Developing a Regulatory Strategy
With a clear understanding of your SaMD’s classification, it’s time to develop a regulatory strategy. This step involves identifying and meeting all the necessary regulatory requirements for your product.
Some key factors related to the software as a medical device approval process you must consider include:
- Applicable Regulatory Pathway
This refers to the FDA regulations that govern your SaMD’s premarket submission process. For example., depending on your product classification, this could be a 510(k) submission or a PMA.
- Regulatory Compliance
This involves ensuring that your product meets all necessary regulatory standards and guidelines. This can include design controls, risk management, and clinical evaluation.
By developing a clear regulatory strategy, you’ll be better equipped to navigate the SaMD certification process smoothly and efficiently.
Moreover, with a comprehensive understanding of these regulatory factors and a clear strategy in place, your development team will be well-equipped to design and develop your software in a way that not only satisfies all necessary regulatory requirements but also aligns with best practices for SaMD creation, fostering a smoother path to market.
As for technical specifications to consider in your strategy, here are the most relevant ones for the SaMD regulatory approval process:
- IEC 62304
This is an international standard for the development of medical software and software within medical devices. It outlines the life cycle requirements for the development of medical software and SaMD, providing a framework for the development, validation, and maintenance of software. - ISO 13485
This is the international standard for a Quality Management System (QMS) for the design and manufacture of Medical Devices. Compliance with this standard demonstrates that your organization has implemented an effective QMS, including processes for continuous improvement and meeting customer and regulatory requirements of the software as a medical device certification. - ISO 14971
This standard pertains to the application of risk management to medical devices. It’s a crucial part of any SaMD regulatory strategy as it provides a framework for identifying hazards, estimating and evaluating risks, and implementing risk control measures. - ISO 27001
This is an international standard for an Information Security Management System (ISMS) relevant to SaMDs that handle sensitive health data. It helps organizations manage the security of assets such as financial information, intellectual property, and employee details. - IEC 62366
This is a process for usability engineering in the development of medical devices. It’s particularly relevant for SaMD as it focuses on optimizing the interaction between users and the system, which can directly impact the safety and effectiveness of the software.
By considering these and other standards in your regulatory strategy (fortunately, not all of them at once), you’re not only working towards compliance but also building a product that is safe, effective, user-friendly, and compliant with the SaMD compliance and regulatory requirements.
Step 4: Testing and Validation: Ensuring Safety, Effectiveness, and Usability
Once you have your regulatory strategy in place, it’s essential to conduct thorough testing and validation of your SaMD throughout the agile development process. This step involves ensuring that your product meets all necessary quality standards, is safe for use, and performs as intended.
Some key factors to consider during this phase include:
- Usability Testing
This involves evaluating how easily a user can interact with the software and how well it meets their needs. Usability testing can help identify any potential design flaws or user interface issues that could impact the safety and effectiveness of your SaMD.
- Performance Testing
This involves evaluating the software’s performance in terms of speed, accuracy, and stability. It’s crucial to ensure your product performs as intended to avoid potential risks for patients.
- Validation Testing
This involves conducting tests to verify that the software meets all functional and non-functional requirements. It’s essential to validate not only the software itself but also any supporting documentation, such as user manuals or instructions for use.
By thoroughly testing and validating your SaMD, you can ensure a high-quality product that is safe for users and compliant with regulatory requirements.
Also Read: Mobile App User Testing: Tools and Best Practices
Step 5: Documenting Your Journey: The Importance of Compliance Monitoring
Throughout the entire development process, it’s crucial to document your journey and keep track of all regulatory requirements and changes adherent to the software as a medical device certification. This not only helps with compliance but also serves as valuable evidence for future audits or submissions.
Compliance monitoring involves maintaining precise records of your SaMD’s development process, software specifications, testing results, risk assessments, and more. This includes potentially anywhere from 10 to 20 different documents, depending on the class of your SaMD and a specific locale’s SaMD regulatory processes.
Some key factors to consider for effective compliance monitoring include:
- Maintaining Accurate Records
This involves keeping detailed records of every step in your SaMD’s development, including design changes and any deviations from your regulatory strategy. This documentation is crucial for demonstrating compliance with medical device regulations and providing evidence of adherence to quality standards.
- Conducting Regular Audits
It’s essential to conduct regular audits of your SaMD’s development process to identify any potential issues or non-compliance early on. These audits can also help ensure that your strategy remains relevant and up-to-date as the software as a medical device approval process evolves.
- Staying Up-to-Date with Regulatory Changes
Regulations and standards are constantly evolving. Hence, it’s crucial to stay informed about any changes or updates to SaMD compliance and regulatory requirements that may impact your SaMD. This can involve actively monitoring regulatory bodies’ websites, attending conferences or seminars, and networking with other medical device developers.
Remember, regulatory bodies like the FDA look closely at these documents during their SaMD clinical evaluation process, and each one aids in demonstrating your product’s safety, effectiveness, and adherence to its SaMD regulatory approval process. Moreover, these records also play a critical role in post-market surveillance and in tracking the ongoing safety and effectiveness of your SaMD once it’s in the hands of users.
Step 6: Completing and Submitting the SaMD Registration Application
Having successfully navigated the prior steps of your SaMD certification journey, you’re now ready to complete your registration application and submit it, along with the necessary fees. This step is crucial, as it officially sets your product on the path to market.
The first part of this process involves completing your SaMD registration application. This entails providing comprehensive details about your software, including its intended use, classification, clinical evaluation data, and evidence of safety, effectiveness, and usability. Remember, transparency and accuracy are key here.
Once your application is complete, it’s time to submit it to the FDA. The FDA offers electronic submission through the eSTAR program, which streamlines SaMD regulatory processes and allows for easier tracking of your application’s status. Do note that there are fees associated with this submission, which vary depending on the type of submission (for instance, a 510(k) or PMA) and the size of your company.
Upon submission, the FDA will review your application. This review process can take some time, but rest assured, the FDA is thorough in its evaluation to ensure all medical devices, including SaMDs, meet their high standards for safety and effectiveness.
Step 7: Post-Market Surveillance
Once your SaMD is on the market, your role in ensuring its safety and efficacy doesn’t end. In fact, it’s just beginning. The FDA mandates post-market surveillance to ensure that your software continues to deliver safe and effective results in its real-world application. This involves monitoring user feedback, analyzing device performance data, and being prepared to make necessary adjustments to maintain compliance.
Here’s an interesting fact: software, unlike traditional medical devices, is dynamic and constantly evolving. New features are added, bugs are fixed, and user interfaces are improved. But did you know that these changes might trigger the need for additional regulatory submissions?
According to the FDA’s guidance document “Deciding When to Submit a 510(k) for a Change to an Existing Device,” significant modifications to a device that could significantly affect its safety or effectiveness or constitute a major change or modification in its intended use, may require a new 510(k) submission. This includes software changes, so it’s important to stay informed about what constitutes a significant modification triggering another round of the SaMD regulatory approval process.
Software as a medical device approval process, therefore, is an ongoing thing, not a one-time goal. It’s like a journey, with each update or modification potentially presenting new regulatory challenges to overcome. But by understanding and meeting these regulatory requirements, you can continue to innovate while maintaining compliance, bringing your healthcare app to market with confidence and keeping it there.
Challenges in SaMD Certification: Common Roadblocks and How to Overcome Them
Navigating the path to SaMD certification can feel like a labyrinth at times. But don’t worry, you’re not alone. Let’s explore some of the common challenges founders face and how you can overcome them.
Implementing SaMD Standards from the Start
Do you think it’s easier to build a house from scratch or retrofit an old one to modern standards? The same logic applies to SaMD development. Rather than upgrading existing software to meet SaMD compliance and regulatory requirements and standards, it’s more effective (and less hair-pulling) to implement these standards right from the start.
Complying with HIPAA Regulations
Remember, HIPAA isn’t going anywhere. Even if your software qualifies as a SaMD, you still need to comply with HIPAA regulations if you’re dealing with protected health information. This includes implementing necessary safeguards and ensuring patient data privacy. Yes, HIPAA-compliant app development may sound like an extra step, but it’s one that guarantees your software’s credibility in the healthcare/ medical device industry.
Utilizing the FDA’s eSTAR Program
Want to speed up your software as a medical device certification process? Consider using the FDA’s eSTAR Program. It allows for the electronic submission of your SaMD registration application and lets you track its status.
Managing Software Updates
In the world of SaMD, updates are a double-edged sword. On one hand, they allow you to improve your software and fix bugs. On the other hand, they can create confusion if different versions of your SaMD exist on the market. To mitigate this risk, urge users to update the app if an old version is detected. It’s a small step that can make a big difference in maintaining software consistency.
Considering Costs for Small Businesses
Good news for small businesses: you typically pay less for 510(k) submissions. This cost consideration can be a game-changer for startups, making the journey to SaMD certification more financially accessible.
Navigating International Regulations
If you’re planning to launch your SaMD in multiple countries, remember that each country has its own set of regulations. This can add complexity to your software as a medical device certification process. However, by conducting thorough research and potentially seeking guidance from regulatory consultants, you can successfully navigate this international maze.
By addressing these challenges of SaMD regulatory processes head-on, you’re not just overcoming roadblocks – you’re paving the way for a smoother journey through the SaMD regulatory approval process.
Learning from Success: a SaMD Case Study
They say success leaves clues. In the world of Software as a Medical Device (SaMD), these clues come in the form of case studies. Let’s delve into one such success story Topflight worked on and glean some valuable insights from it.
Case Study: JOOVV
Imagine an IoT red light therapy machine that can provide a myriad of health benefits right from the comfort of your home. Too good to be true? Not if you’re talking about Joovv.
Joovv is a shining example (pun intended) of a SaMD product done right. The journey wasn’t easy, but with a clear vision and diligent execution, Joovv achieved its goal. So, how did they do it? How did we help them navigate through the software as a medical device approval process?
Firstly, they realized the importance of IEC 62304 compliance. This standard, which pertains to the life cycle requirements of medical device software, was a critical aspect of their product development process. To ensure compliance, Joovv shared responsibilities between their architect and ours.
Secondly, Joovv didn’t overlook HIPAA regulations. They implemented necessary safeguards to ensure patient data privacy. This was a crucial step in building their credibility in the healthcare industry.
Lastly, Joovv built their IoT mobile app from scratch. By doing so, we were able to incorporate SaMD compliance and regulatory requirements from the get-go rather than trying to retrofit them into existing software.
The result? A successful SaMD product that’s now being used by professional athletes, including the San Francisco 49ers. Talk about a touchdown!
Lessons Learned from Successful SaMD Certifications
Joovv’s success story offers valuable lessons for aspiring SaMD developers. Here are a few key takeaways:
- Start with a clear vision: Know what you want your SaMD product to achieve, and let this vision guide your healthcare app development process.
- Don’t overlook regulatory compliance: Both IEC 62304 and HIPAA are crucial in the SaMD space. Ensure your product aligns with these standards.
- Build from scratch: It’s much easier to incorporate SaMD standards into a new software than to retrofit an existing one.
- Collaborate effectively: Sharing responsibilities between different teams can help ensure all bases are covered.
Remember, success in the SaMD space isn’t just about having a great idea – it’s about executing that idea in a manner that aligns with the SaMD regulatory approval process and meets user needs. And as Joovv has shown, when you get these elements right, the sky’s the limit!
I’d be happy to share more, but I can only mention we’re helping an MSK + AI solution from our portfolio through the SaMD certification process.
Looking Ahead: The Future of SaMD Certification
As we journey forward into the digital age, the landscape of Software as a Medical Device certification continues to evolve. With the market projected to expand at a 52% CAGR, it’s clear that SaMD is becoming an integral part of healthcare’s future. But what does this future look like? Let’s delve into the emerging trends and the role of AI/ML and Big Data in shaping SaMD’s trajectory.
Emerging Trends in SaMD
One significant trend in SaMD is the FDA’s Software Pre-Certification Program. Aimed at streamlining the regulatory process for digital health technology, this program is a testament to the FDA’s commitment to fostering innovation while ensuring patient safety.
Another emerging trend is the increasing importance of IEC 62304 compliance. As SaMD developers continue to navigate the regulatory maze, training on the requirements of this standard is becoming increasingly crucial.
The FDA acknowledges the unique challenges of AI-based SaMD solutions, which evolve over time. Traditional approaches to SaMD regulatory processes may not apply. The FDA is developing a tailored regulatory framework for AI and machine learning-based SaMD, allowing modifications while ensuring patient safety. This recognizes the rapid evolution of the SaMD space and the need for adaptive regulatory frameworks.
The Role of AI and Big Data in SaMD’s Future
Artificial Intelligence (AI) and Big Data are no longer just buzzwords – they’re game-changers in the SaMD space. The FDA recognizes this and has outlined guiding principles for Machine Learning-enabled medical devices.
For instance, the FDA encourages the use of predetermined change control plans for these devices. These plans outline anticipated modifications, thus ensuring continuous alignment with regulatory requirements even as the software evolves.
Moreover, the FDA underscores the importance of “Good Machine Learning Practice” (GMLP) in SaMD development. By adhering to GMLP, developers can ensure their AI-powered SaMDs are reliable, safe, and effective and are ready for the software as a medical device approval process.
According to the FDA, there has been a noticeable deceleration in the year-over-year growth of AI/ML-enabled devices. In 2021, the increase stood at 15%, followed by a further decline to 14% in 2022, after experiencing a substantial surge of 39% in 2020 compared to 2019. However, despite this temporary slowdown, projections indicate a significant resurgence in 2023, with the expected increase of AI/ML-enabled devices surpassing 30% compared to the previous year. This data reinforces the undeniable upward trend of AI/ML in Software as a Medical Device (SaMD). [Partial disclosure: We’re currently handling the SaMD clearance for one of our clients’ AI health products.]
As we move forward, the integration of AI/ML and Big Data in SaMD will continue to transform healthcare delivery. From improving diagnostics to personalizing patient care, the potential is immense.
As you can see, the future of SaMD certification is dynamic and exciting. As regulatory frameworks adapt to technological advances, developers have the opportunity to create groundbreaking software products that can revolutionize healthcare. Are you ready for the journey? Reach out to our expert to discuss how SaMD compliance and regulatory requirements apply to your health app.
Frequently Asked Questions
What exactly is Software as a Medical Device (SaMD)?
SaMD refers to software intended for medical purposes without being part of a physical medical device. This could range from an app that helps manage a chronic condition to software that aids in diagnosing illnesses.
What does the regulatory approval process for SaMD entail?
The regulatory approval process involves ensuring your SaMD product complies with relevant regulations and standards, often involving extensive documentation, thorough testing, and careful submission of required information to regulatory authorities like the FDA.
How can I master the regulatory approval process for SaMD in healthcare tech?
Mastery comes from a deep understanding of the applicable rules, staying updated with regulatory changes, adhering to industry best practices, and seeking advice from experts when needed. The software as a medical device certification is a complex journey, but with the right knowledge and resources, it’s certainly one that can be navigated successfully.Remember, at the heart of SaMD is the potential to revolutionize healthcare delivery, making it more accessible, effective, and personalized. The SaMD regulatory approval process, while challenging, plays an integral role in ensuring these innovative solutions meet the highest standards of safety and efficacy. Isn’t it incredible to envision the impact we could make by navigating this landscape effectively?